Unapproved medical claims are widespread in Amazon’s hemp market, according to an analysis by CBD Oracle.
Although Amazon ostensibly bans CBD, the “hemp” products advertised on the platform promise the benefits of CBD and even list large dosages in milligrams to create the perception that you’re buying something legitimate. But how do these products discuss their effects and possible benefits, and is this allowed under US law?
We performed a detailed analysis of 56 Amazon hemp product pages to find out.
TL;DR
- The FDA lists 10 criteria to determine whether a claim is an allowed structure/function claim or a disease claim needing pre-approval.
- 52% of Amazon hemp products make such a disease claim, according to CBD Oracle’s analysis.
- Almost half of these are made through images implying pain or inflammation relief.
- These claims risk deceiving consumers and may even discourage them from seeking genuine medical help.
The FDA Guidance: Structure/Function Claims vs. Unapproved Medical Claims
The line between an allowable claim and an illegal one can be quite fine, but there is clear guidance from the FDA to help companies stay on the right side of the law.
These guidelines come in the form of 10 criteria, which distinguish an allowable “structure/function” claim from a “disease claim” that requires FDA approval.
If it was possible to distill the 10 criteria down into a single rule, it might be something like “making general claims about the known benefits of vitamins and nutrients for healthy people is fine, but identifying a specific disease (other than vitamin deficiencies) that is helped by a specific compound in your product is likely a disease claim.”
So this means that saying “calcium helps build strong bones” is fine but saying “CBD alleviates depression” is a disease claim.
The first example just re-states a widely-known and general fact about calcium, about a benefit that applies to any healthy adult and not just someone with a specific issue, while the second identifies a medical condition (depression) and a treatment (CBD).
The details here are important, and while we won’t go into all of them here, a few important points for this analysis are:
- Claims about characteristic symptoms of a disease are basically treated the same as claims about a disease. The FDA uses the example of “reduces cholesterol” being characteristic of cardiovascular disease and so probably being a disease claim. This is part of criterion 2.
- Pictures of abnormal tissues or organs can constitute disease claims, depending on context. Generally, if you have red highlighting or something that identifies an “abnormal” state, it’s likely to be a medical claim. This is part of criterion 4.
- Claiming that a product is a substitute for a treatment for a disease or has fewer side effects is generally considered a disease claim. This is criterion 6.
52% of Amazon Hemp Products Make an Unapproved Disease Claim
Based on CBD Oracle’s analysis, 52% of Amazon hemp products appear to make a claim that violates FDA rules.
The most common reason for this was using images that could constitute a disease claim, such as a red-highlighted organ or painful joint. In total, 29/56 products made an unapproved disease claim, and 12 of these did so through imagery, usually implying that the product helps with pain or inflammation.

Other products clearly made comments relating to illnesses, such as this example which claims that the hemp oil, vitamins and folic acid in the product boosts your immune system, and is “especially important during cold and flu season, or when you’re feeling run down.”
Products intended for pets were even more blatant, with this example explicitly saying it “reduces seizures.”
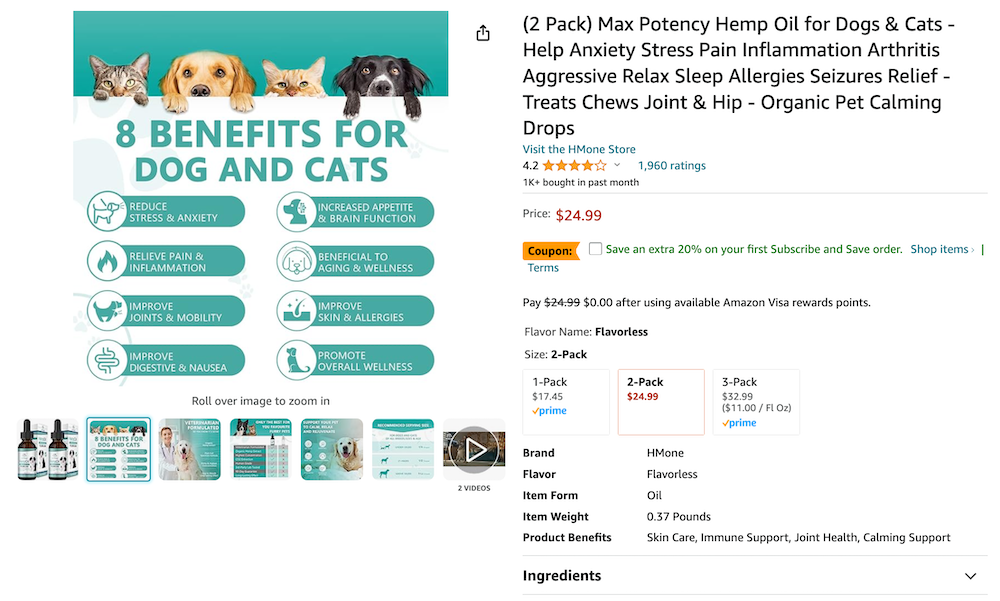
It’s important to note that we are not lawyers, and are not definitively saying these products break the law – we could be wrong. However, we reported the 29 offending products to the FDA on the grounds that they appear to clearly violate the guidance on allowed claims.
How Does This Affect Consumers?
If you’re reading an article on CBD Oracle, it’s likely that you wouldn’t personally be taken in by the widespread medical claims about the hemp products on Amazon. We have many articles discussing the actual benefits of CBD and what we know so far, and say at basically any opportunity that you shouldn’t buy a CBD product if it doesn’t have a lab report (COA) available.
But for all the people out there with a passing interest in CBD, who may pick up a bottle of gummies from Amazon out of curiosity, the misinformation could do genuine harm. If you don’t know what CBD can and can’t do, these claims may appear to be legitimate, and at worst, they could convince someone to forgo treatment which is proven to be effective.
The FDA expressed their concern about this issue when we spoke to them for the report:
“The FDA continues to be concerned about products that are sold online and in stores for treating or preventing diseases or conditions but that have not been reviewed for safety and effectiveness by the FDA. […] This deceptive marketing of unproven products also raises significant public health concerns because patients and other consumers may be influenced to use unapproved products rather than treatments with scientifically proven benefits to treat serious and even fatal diseases.”
Even if the condition being treated is not particularly serious or life-threatening, claiming that your product helps people suffering from it – when it likely does not – makes it more likely that they will spend their money on it.
However, the majority of products in our analysis didn’t contain any cannabinoids at all. So even if it’s not physically dangerous in some cases, the companies are still profiting from deception at a time when people across the US and worldwide are struggling with rising costs of living.
CBD and hemp have many benefits, and we’d recommend them to a whole host of people for various reasons. However, there is no excuse – under any circumstances – for misleading people with health conditions just to make a bit of money.
The “hemp” on Amazon is nothing more than modern snake oil.
Related:
- Study Reveals That 11% of Hemp Gummies on Amazon Contain THC
- Amazon Could Get Fined Up to $1.3 Million Over INFORM Consumers Act Violations
- Half of the Hemp Product Listings on Amazon Make Illegal Medical Claims
- Amazon’s Hemp Market Costs the CBD Industry Over $60 Million Per Year
- This Gummy on Amazon Says It Has 200,000,000 mg of Hemp Extract, But Is That Even Possible?
- You Could Make $100k From Selling Hemp on Amazon: Here’s How
- Hemp Products on Amazon Are Full of Fake Reviews
- China-Sourced Gummies Are Making Amazon’s Hemp Market Even Worse
- Here’s Why You Shouldn’t Buy CBD on Amazon (And What You Should Do Instead)
References
- Nutrition, C. F. F. S. a. A. (2017, November 10). Small Entity Compliance Guide on Structure/Function Claims. U.S. Food And Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-structurefunction-claims
- The Food and Drug Administration (February 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/FDA-comments-Amazon-hemp-market.pdf

