If you ask Amazon, they’ll tell you confidently that they don’t allow CBD gummies on their platform. But if you scratch the surface just a little, you’ll find a plethora of products backed by stock photos of happy young people proudly proclaiming that they contain “10,000,000 mg” or some similarly ungodly amount of hemp extract. They might not say “CBD” on the product page, but the messaging is crystal clear to anybody with a passing knowledge of hemp.
But what do these products actually contain? Do any of them make medical claims? Are they breaking any laws? And perhaps most importantly, why has Amazon so spectacularly failed to prevent the listing of products obviously in violation of their terms of service?
To answer these questions, CBD Oracle purchased 56 hemp products sold on Amazon.com and sent them to InfiniteCAL Labs for intensive analysis to find out whether the extreme claims match up to reality. We spoke to legal, economic and scientific experts, hemp companies with experience selling on Amazon and even the US Food and Drug Administration (FDA). We also attempted to get comments from every single one of the fly-by-night companies whose products we tested.
The short version is that Amazon’s self-proclaimed hemp products are almost entirely unreliable, with no accountability for the sellers, widespread unapproved medical claims, many violations of federal law and no hemp at all in over a third of products tested.
Read the full report below, or download the summary here (PDF).
Table of Contents
The Basics: Why CBD Is (Mostly) Banned on Amazon
The Lab Test Results: What Does Amazon “Hemp” Actually Contain?
The Marketing of Amazon’s Hemp Products
What Is It Like to Sell Hemp on Amazon?
What’s It Like to Buy Hemp on Amazon?
The INFORM Consumers Act (And How Amazon Is in Violation)
It’s Not Just Amazon: eBay, Walmart, and Alibaba
Is Amazon’s Poor-Quality “Hemp” Undermining the Whole Industry?
How to Improve the Situation for Hemp on Amazon (and Other Marketplaces)
Executive Summary
- Amazon policy explicitly forbids CBD, unless it’s a topical CBD product specifically approved by Amazon.
- We purchased 56 of the most popular hemp products on Amazon and sent them to InfiniteCAL Labs for testing.
- 30% of products tested were confirmed to contain CBD by lab testing.
- 11% of products tested positive for THC, including three containing delta-8 THC at potencies of up to 76 mg per gummy.
- 62.5% of products tested had no cannabinoids at all, and gas chromatography testing shows that 43% don’t contain any hemp at all.
- 96% of products did not provide an accurate dosage.
- Safety testing of 5 products for pesticides, solvents, mycotoxins, microbials, heavy metals and foreign matter revealed no issues.
- Products regularly advertised absurd dosages that ranged from simply inaccurate to physically impossible. For example, advertising 7.7 pounds of “hemp” in a 0.8 pound container.
- 52% of product pages made an unapproved medical claim about their products.
- 95% of products do not include a certificate of lab analysis (COA), and none of the ones we found were available via Amazon.
- 89% of products came from manufacturers that did not respond to inquiries via phone or email.
- About half of the products did not have reliable customer reviews, had suspicious spikes or declines in the number of reviews, or were rated untrustworthy by Mozilla’s FakeSpot.
- Sellers with experience working with Amazon report that enforcement is inconsistent and complain about less honest companies benefiting from the current system.
- CBD Oracle reported several cases of flagrant false advertising to Amazon but the products still remain on the storefront.
- We identified 28 products whose listings appear to violate the INFORM Consumers Act.
- CBD Oracle reported all products making medical claims to the FDA, and all breaches of the INFORM Consumers Act to the FTC.
- Amazon could get over $1.3 million in fines from INFORM Consumers Act violations.
- We estimate the value of Amazon’s hemp market at $64 million per year, although it could be even higher than this.
- Not only do these unreliable hemp products take revenue that would otherwise go to reputable companies, but they also harm the reputation of the hemp industry overall.
- Amazon should immediately remove all products making unrealistic dosage claims or unapproved medical claims.
- Removing the prohibition on CBD would enable reliable companies to fairly compete in the marketplace, and ensuring every product has a COA from an accredited lab would hugely improve the quality of hemp sold.
The Basics: Why CBD Is (Mostly) Banned on Amazon
Amazon’s policy on CBD is that it is prohibited aside from “certain CBD topical products” that have been confirmed to comply with applicable laws, regulations and policies.
Specifically, the policy states that:
Listings for products containing cannabidiol (CBD) are prohibited, including but not limited to:
- i. Full spectrum hemp oil
- ii. Rich hemp oil
- iii. Products that have been identified as containing CBD by LegitScript
In a sense, this is understandable. The legality of CBD products was established clearly in the US by the 2018 Farm Bill, but there are still many specific cases that raise issues and state-level rules can vary substantially. Even ignoring state laws, the federal Food, Drug and Cosmetic Act (FD&C Act) clearly makes it illegal for a CBD edible product to be sold interstate, for instance.
The US Food and Drug Administration (FDA) explained the legal situation around the FD&C Act to CBD Oracle:
“Under the Federal Food, Drug, and Cosmetic Act (FD&C Act), any product intended to have a therapeutic or medical use, and any product (other than a food) that is intended to affect the structure or function of the body of humans or animals, is a drug. Drugs must generally either receive premarket approval by the FDA through the new drug application (NDA) process or, for certain nonprescription drugs, meet the requirements for legal marketing without an approved drug application in section 505G of the FD&C Act. With limited exceptions not applicable to CBD and other cannabis-related products, an unapproved drug cannot be distributed or sold in interstate commerce, as they are not approved by the FDA for the diagnosis, cure, mitigation, treatment or prevention of any disease. Consumers should beware of purchasing and using any such products.
CBD also cannot lawfully be added to food. This is true for foods for humans and animals. We have issued warning letters to companies for illegally selling foods with CBD. Foods with undeclared CBD content pose a risk of unintentional or excessive CBD consumption.”
From this perspective, Amazon is making a sensible business decision. They have plenty of income sources, so why open yourself up to issues by allowing sellers to offer potentially illegal products? They make exceptions for non-consumable products in rare cases, but otherwise the policy is “no CBD on Amazon.”
The “Hemp” Sold on Amazon
The problem is that it is incredibly easy to find CBD on Amazon, even if they call it “hemp” without specifying what hemp components it actually contains. All it takes is a little edit to your labels (whether physically or digitally) and some careful wording and it’s apparently not a challenge to sell CBD on Amazon at all.
The crucial trick is just never mentioning CBD in your product description. If you call it “hemp” then it could be many things which are not banned by Amazon (such as hemp seed oil), and the marketplace is full of these products. However, if you combine this with information about dosage and a list of medical benefits of CBD, savvy consumers will put two and two together.
With that workaround, the hemp market on Amazon is superficially like the market outside of Amazon. They have gummies, oils and candies, topped off with reviews discussing the effects (or lack thereof) and the products’ benefits for issues such as getting to sleep. If you haven’t bought CBD often before, and if you haven’t looked at recommendations and advice on sites like CBD Oracle (for instance, don’t buy unless you see a lab report), then everything might seem totally legit.
But the reality is very different. We spoke to several sellers with experience selling on Amazon, and all essentially had the same complaints. Jan Brandrup, CEO of Neurogan, commented that:
“The so-called ‘hemp products’ on Amazon are not related to actual hemp. They use the name as a sales tactic, without any check or control. It’s alarming how easily consumers are deceived into trusting these products, just because they are sold on a reputable platform like Amazon.” Adding that, “the best case is they may drain your wallet.”
Kelly Lombard, founder of Forge Hemp, commented to CBD Oracle that, “I’m very concerned about the quality and content of hemp products offered by brands that don’t physically exist outside of Amazon. There’s no quality control by Amazon and no accountability to any state hemp regulations or U.S. manufacturing standards. Amazon’s super power has always been to connect U.S. consumers with overseas manufacturers. That works for hard goods, but I believe there should be higher standards for products coming into the country that are intended for human consumption.”
Mike Sill, CEO and co-founder of Sunday Scaries, agreed, “The quality of hemp products on Amazon is very low. When you search for ‘CBD gummies’ on the platform, no reputable brands populate in your search results. The reason for this is that credible brands like Sunday Scaries, Charlotte’s Web and cbdMD are not allowed to sell on Amazon without being banned.”
“The companies that you see as a consumer are engaging in a process called ‘Brand Burning.’ This means that if they get banned on Amazon, they’ll simply burn their label, whip up a new label, slap it on the same exact product and then reupload it to Amazon. Their business model doesn’t include a focus on building a reputable brand and providing the highest quality and safest products to consumers, they are just looking for a quick sale and will do whatever is necessary to stay ‘live’ on Amazon.”
These products only exist because they were apparently able to “trick” the monitoring system into thinking they don’t contain CBD. But this creates a huge issue: customers often have no information about what the product they’re buying actually contains. Hemp is a plant that contains hundreds of cannabinoids, and even with the subtle suggestions that you’re buying CBD oil, for example, there is no explicit promise that it contains any cannabinoids at all.
The Lab Test Results: What Does Amazon “Hemp” Actually Contain?
To see what is actually in these products, CBD Oracle bought 56 of them and sent them to InfiniteCAL Labs for testing. While we tested a small number for safety purposes, the main goal was working out what – if any – cannabinoids they contained, whether they breached Amazon’s stated policy and whether consumers were getting anything close to the advertised dosages.
The vast majority of products (80%) were gummies, 8 were tinctures, 2 were topical creams and the last one was a pack of mints. Additionally, 89% made a specific numeric claim regarding their dosage.
30% of Amazon Hemp Products Contain CBD
Around 30% of all products tested contained CBD. This means that they are giving customers what they likely expect from their purchase in a basic sense, but also means that they are breaking Amazon’s policy and that the gummies were in violation of the FD&C Act.
To be specific, 17 out of 56 products were determined to contain CBD by lab testing, with an average amount of 547 mg per package. Specific products varied quite a lot too, with a minimum of 28 mg of CBD per pack and a maximum of 1,582 mg, ignoring the many products that didn’t contain CBD at all. On one hand, these products at least contain hemp in a meaningful sense, but on the other, these products explicitly break Amazon’s policies and should never have been available on the marketplace.
Moreover, if Amazon’s primary concern is staying legally compliant, it’s worth noting that section 301(ll) (21 U.S.C. 331(ll)) of the FD&C Act makes it illegal to sell any edible product containing CBD across state lines.
Some Products Contain Delta-8 and Delta-9 THC

It should go without saying that THC is also banned from sale by Amazon’s policy. However, six products contained THC, and the three that contained the most were actually primarily delta-8 THC.
In total, 11% (6/56) of products tested positive for THC, but all of these were under the Farm Bill limit for delta-9 THC. However, when you include delta-8, three products had very high quantities of THC: 641, 2,507 and 3,028 mg per pack. The highest of these works out to a massive 76 mg of THC per gummy.
Not only does this obviously break Amazon’s policy, such delta-8 THC products are illegal to sell in many states. Additionally, in regulated cannabis edible markets, products can generally only have up to 100mg per package of delta-9 THC. Even if we account for the fact that delta-8 is only estimated to be 50 to 75% as potent as delta-9, all of these are likely more potent than bona-fide cannabis edibles.
Over a Third of Products Contain No Hemp At All
The majority of the sampled hemp products didn’t contain any cannabinoids and over a third had no hemp at all. Simply put, if you buy “hemp” from Amazon it is likely that you will actually be buying an expensive jar of gummy bears. Gelatin and sugar, priced at a premium.
Firstly, ordinary cannabinoid potency testing showed no cannabinoids at all in 62.5% (35/56) of products we purchased. In comparison to the promised hemp extract content, where companies advertised at minimum 150 mg of hemp extract.
However, the wording (“hemp extract” not “cannabinoids”) might suggest that they meant “any hemp component” rather than cannabinoids specifically.
Dr. Erik Paulson, Lab Manager at InfiniteCAL, explained to us that, “Hemp is typically infused into consumable products in one of two ways:
- The hemp seeds, which contain no cannabinoids, are either included whole or pressed to extract the hempseed oil, or
- Extractable material is pulled out of the leaves, stems and/or buds using some solvent or solventless extraction technique, and generally further processed to isolate the cannabinoid/cannabinoids of interest.
If the second method is taken, it is usually performed in order to make a product that contains cannabinoids. If products are being made from hemp seeds or hempseed oil, then the product should not contain cannabinoids (unless there were some contamination issues during the harvesting of the seeds). So it certainly is possible that we could see ‘hemp infused products’ not containing cannabinoids, but they would then have to contain hemp seeds or hempseed oil.”
With this in mind, we got InfiniteCAL to run GC-MS-MS (i.e. gas chromatography-mass spectrometry) testing to find out whether this was the case, because this method essentially looks at everything in the product, not just specific components you would expect to see. The results showed that 24 products (43%) contained absolutely no hemp at all.
Erik added, “We did run some tests however to see if we could see evidence of the presence of hempseed oil, and in only one of the samples we tested we saw evidence that hempseed oil was present. If there are no cannabinoids, hemp seeds, or hempseed oil in the product, then what else could they be putting in there that could still be considered hemp?”
We intend for this to be an objective report, so we would usually shy away from making any statements like this. However, in these cases it is 100% clear from the testing: these companies are lying to you.
Almost No Products Had Accurate Dosage Labeling
Perhaps unsurprisingly, given the sometimes extreme dosage claims made by the companies, 96% of products did not advertise an accurate dosage.
If we assume the dosage listing refers to cannabinoids (and not just the total mass of hempseed oil), just two products were confirmed by lab testing to have a dosage within 10% of that listed on their labels. They contained an average of just 25% of the advertised dosage. In most cases, this was less than advertised, but one product primarily containing delta-8 THC had twice the promised dosage.
A Small Subset of Products All Passed Safety Testing
For the first five products we tested, we also had InfiniteCAL perform safety testing to look for things like microbial contamination, residual heavy metals, mycotoxins, foreign matter and pesticides.
These tests were all passed, and based on our experience testing both delta-8 and hemp delta-9 products (and the apparent lack of hemp in many cases), we didn’t commission safety testing on the remaining products. All of the hemp products we’ve ever tested through three investigations have been clean, and most of these products don’t even have hemp.
The Marketing of Amazon’s Hemp Products
Perhaps unsurprisingly, the lab results reveal products which largely do not offer what they claim to and in many other cases, break Amazon’s policy. However, the problems with the hemp market on Amazon are obvious before you even purchase a product.
How Much Hemp Can You Fit Into a Gummy?
The most obvious and glaring issue with Amazon’s hemp market is that it is chock-full of claims which cannot possibly be true.
One example product from GummiMi is a perfect example, but far from the worst. The total “item weight” of the product is listed as 6.74 ounces (0.42 pounds), which is equivalent to about 191 grams. This same product claims to contain “15,000,000 mg” of well, something. The problem is that 15,000,000 mg is 15,000 grams, almost 80 times the stated weight of the whole product.
This means that before we even get into the issue of whether this product contained hemp at all (it didn’t), the claim printed front-and-center on the label is completely impossible. You cannot fit 33 pounds of stuff into a container and have the total weight be 0.4 pounds.
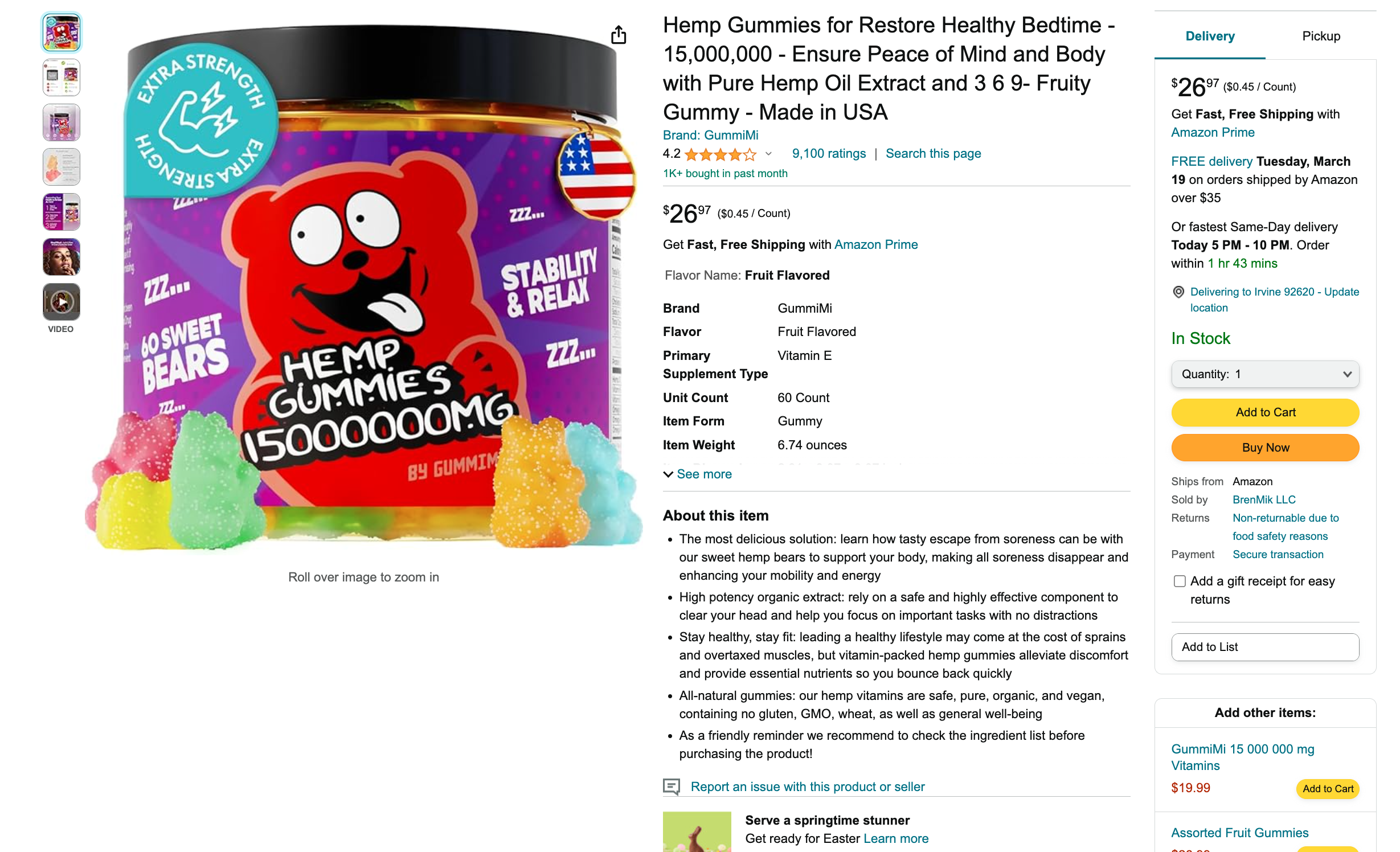
We asked Dr. Erik Paulson about this confusing trend, “Yeah, that boggles my mind too. Even a product labeled at 1,000,000 milligrams equates to 1 kilogram, which is 2.2 pounds, which is clearly way more than the mass of the product itself. I think some people do recognize this issue, as you can read in some of the reviews for these products, but surely they are taking advantage of people who just aren’t thinking about it enough, or are unfamiliar with the metric system. I think it also may be that the manufacturers themselves are just trying to one-up their competitors, so they put bigger and bigger numbers on the label until they’ve reached a point where it’s physically impossible for a product to contain that amount of ‘hemp.’”
Kelly Lombard from Forge Hemp agreed, adding “Amazon’s own guidelines prohibit products from making false or misleading claims, and these products are an easy example of such.”
Simply put, these products make impossible claims because they expect people to not know any better.
The highest amount of cannabinoids in any gummy as confirmed by testing was 77.2 mg per gummy, with this working out to about 1.5% of the total weight. Mike Sill from Sunday Scaries explained that this would be at the upper end of what is possible:
“A gummy with any gelling base, whether it be gelatin or pectin, has a threshold for how many additives it can support before it loses its ability to gel and loses its structure. […] Even if you could get a 20+ gram gummy to fit 5 grams of extract and still gel properly, it would taste like battery acid and be the size of a golf ball.”
Concluding that, “In short, 1-2% of the gummy can be hemp extract without interfering with the gelling matrix (which is on the high end). 0.2% – 0.8% seems to be the norm. Anything higher than that is going to be really bitter and a typical 2-3 gram gummy bear cannot possibly fit 5 grams of extract.”
What Are You Buying? It’s All About the Implication
The GummiMi product discussed in the previous section may have made an unrealistic claim anyway, but in reality it didn’t explicitly say that the 15,000,000 mg figure was referring to any particular hemp component or even hemp extract overall.
If you were trying to defend this product, you might argue that they didn’t really say that it was hemp or CBD or anything specific, so it isn’t really false advertising.
Mike Sill spoke about similar tactics, “Most of the fake brands on Amazon pushing extremely high mgs (100mg+ per gummy) make these claims by either adding up the total weight of the entire unit (including the packaging) and listing it in bold letters to confuse the customer, or they are flat out falsely advertising.”
Even leaving aside the physical impossibility of some of these claims, in most cases a combination of factors strongly implies that the claim refers to CBD or another hemp cannabinoid. Look at this part of the description from the same Hooloo product:
“Hemp oil infused gummies to help you fall asleep and stay asleep, say goodbye to restless nights. Wake up feeling refreshed, add a moment of focus and energy in the day time; Peace, calm and bedtime support in the night.”
Now, with the combination of the specific dosage given and these supposed effects, any informed consumer would conclude that these hemp gummies have CBD, CBG or other cannabinoids that could produce these effects. You don’t have to say it does, because the implications created by the product page put this idea in the minds of potential customers anyway.
As Andrew Livingston, Director of Economics and Research at Vicente, captured the issue perfectly in a comment to CBD Oracle:
“The products clearly insinuate they are cannabinoid rich with terms like ‘45,000,000 mcg per bottle,’ ‘Maximum Strength,’ and qualitative statements like ‘Great for Peace & Relaxation’ and ‘Overall Health Enhancement.’ But it is not clear if these products are made from pressed hemp seed oil which likely contain no cannabinoids and have been used in supplements even before the 2014 Farm Bill or were produced from hemp flower resin grown for its cannabinoids.”
What Does 200,000,000 Mean Without Units?
Even if a consumer is lucky enough to choose a product that even contains any CBD, like this one from Advanced Bionetix, the “dosage” listed often tries to side-step the issues discussed above by omitting any type of unit from the overly large number.
In this case, that number is 200,000,000. But is that milligrams (mg)? Micrograms (mcg)? Maybe it’s nanograms (ng)? There is no answer to this question because there was no unit given. These three options alone span the range from 200 kg right down to 200 mg.
This gives a customer zero certainty about what they’re even being offered; it’s just a big number with no context and no correlation to the actual contents of the product. The lab results show that this product contained around 520 mg of CBD per container, with no other cannabinoids found. None of the three options discussed above would make this “accurate” in any way.
However, given the industry norm of showing dosages in milligrams (i.e. 1/1000 of a gram), most consumers will undoubtedly assume this is the meaning if no unit is given. Micrograms are already a little obscure and no hemp product we’ve ever encountered would express their dosage in nanograms. Again, this is a case where the deception isn’t explicit, but the assumptions of customers are allowed to fill in the gaps.
Most Products Likely Make Unapproved Medical Claims
Along with a general lack of accurate information regarding dosage, our analysis shows that 52% of the hemp products we purchased from Amazon appear to make an unapproved medical claim.
CBD Oracle asked the FDA for comment on the issues covered in the report, and the statement they gave opens by clearly addressing this issue:
The FDA continues to be concerned about products that are sold online and in stores for treating or preventing diseases or conditions but that have not been reviewed for safety and effectiveness by the FDA. Selling unapproved products with unproven claims is a violation of the law, and puts patients at risk, as these products have not been proven to be safe or effective.
The U.S. Food and Drug Administration
The FDA’s guidance on this issue comes in the form of 10 criteria, which are explained well by the law firm Venable in this document. In short, while manufacturers may be allowed to make “structure/function” claims – for example, “calcium builds strong bones” – they cannot make what the FDA calls “disease claims.” These are claims that a product can help in the diagnosis, mitigation, treatment, prevention or curing of a disease.
We analyzed each product listing based on the FDA’s criteria, using both FDA guidance and the informative document from Venable as a guide. Note that we are not lawyers and we are not saying that these are definitively unapproved health claims. However, what we are saying is that based on the criteria given by the FDA and the plain meanings and implications of the product descriptions, we have judged that these statements meet the criteria and have accordingly reported them to the FDA for final determination.
In total, 29/56 products make a claim that meets the FDA criteria for a disease claim. These varied from the more blatant examples through to more mundane ones where a better choice of graphic could have cleared the manufacturer.
For example, this hemp oil from GummiMi claims:
- “Promotes rеlief from various disсоmfоrts, including jоint and musclе аchеs, and hеаdаches”
- “Reduces аnxiеty & strеss”
- “If you always deal with pаinful joints, aching muscles, and inflаmmation, using of this product can help free you from discomfort, allowing you to be more productive and achieve all your work goals.”
In these examples, the product identifies disease states (e.g. anxiety, stress, inflammation, pain) and claims that the hemp oil will provide relief from these issues.
Likewise, this example from Stances Orchard states:
“The combination of hemp oil extract and vitamins and folic acid can help boost your immune system. This can be especially important during cold and flu season, or when you’re feeling run down.”
Again, this identifies both specific conditions (cold and flu) and claims that the hemp oil extract, vitamins and folic acid will help to prevent or even relieve these conditions.
While there are many claims like this, the most common issues identified related to images used in the marketing materials. For example, this product from HMone debatably strays close to medical claims by saying it “may relax muscle & relieve joint pain” but it also says “Support cardiovascular” above an image of a heart with an EKG tracing. The FDA describes the issue with this imagery in their guidance:
“Some symbols, like the heart symbol, are so widely recognized as symbols for disease treatment and prevention that their use is ordinarily an implied disease claim. Symbols such as EKG tracings are also implied disease claims because they are strongly associated with heart disease […]. It would be an unusual circumstance in which the use of these two symbols would not be implied disease claims.”
More common, though, is many products showing red-highlighted joints, organs or skin which the FDA considers to be an implied disease claim:
“For example, pictures of healthy organs would constitute an appropriate structure/function claim while a picture of an abnormal tissue or organ would be an implied disease claim.”
In total, we identified 12 cases of this type of imagery. Some typical examples can be found from brands such as NLMUBR LUCKSIT, Feelgo, Healing Drops and Healthergize. Generally, these involve a knee or back with red highlighting or even lightning-like “pain” lines that imply “abnormal tissue” visually, even if the text itself doesn’t necessarily say this.
While many cases are somewhat blatant, it must be said that the products intended for pets involved the most obvious examples of medical claims, such as this product from Wachray which explicitly says that it reduces seizures in pets.
In their statement to CBD Oracle, the FDA also pointed out the inherent risk from unapproved claims, “This deceptive marketing of unproven products also raises significant public health concerns because patients and other consumers may be influenced to use unapproved products rather than treatments with scientifically proven benefits to treat serious and even fatal diseases.”
Overall, some products toed the line carefully and were clearly the result of legal advice regarding which claims were acceptable and which were not. However, other product descriptions read like they heard this same advice but the message was misapplied, misunderstood or misquoted, like they’re the kid at the end of the line in a game of Telephone.
95% of Hemp Companies on Amazon Don’t Provide a Certificate of Analysis to Consumers
Only three companies provided Certificates of Analysis (COAs) to consumers, and none of these were visible, linked to or available from the product page on Amazon. This amounts to almost 95% of products not having COAs available at all.
The COA has become an important marker for quality hemp products, to the point that virtually all credible companies offer them to consumers and many states require it for any hemp products. Websites like CBD Oracle do not recommend buying any product that has not been verifiably tested by a lab. Simply, this is the only way CBD and hemp customers can realistically verify what it is that they’re buying.
In addition to searching the companies’ websites and the Amazon listings for COAs, we also emailed every single company with contact information available to request COAs and ask for other pertinent information about the product (for example, whether the hemp was US-sourced). No companies had responded at the time of writing. This means that even particularly engaged customers, willing to personally request this information, would not receive the information.
The only positive is that 2/3 of products that actually provided a COA were also tested for safety and not just potency.
What Is It Like to Sell Hemp on Amazon?
Based on the lab testing and the misleading or non-existent information given to consumers, it’s clear that Amazon’s hemp market is far from the most reliable around. However, the huge reach of Amazon makes it an appealing option for CBD and hemp sellers looking to reach a bigger market. But how is it for the sellers in practice?
We asked three Amazon sellers, Mike Sill, CEO and co-founder of Sunday Scaries, Kelly Lombard, founder of Forge Hemp and another hemp seller who wished to remain anonymous, about their experience.
Mike Sill was very clear in his response:
Our experience selling hemp on Amazon has been miserable, and we would not recommend it to other hemp brands. There’s a reason that the top CBD companies, including the publicly traded ones, do not advertise on Amazon and it’s because our accounts will be banned.
Mike Sill, CEO and co-founder of Sunday Scaries
Adding, “The worst part about it is that Amazon lets these illegitimate companies write in the names of credible CBD brands, like Sunday Scaries, in their product descriptions. They are keyword stuffing competitor brand names within their product placements to capture the search intent of unknowing customers. These illegitimate companies are also brand bidding on sponsored Amazon ads using the same tactics. So, if a Sunday Scaries customer wants to find our products on Amazon and searches for them, they’ll instead find brands impersonating us that are solely trying to capture this search traffic.”
Kelly Lombard was more positive, “Our brief experience reaching a national audience through Amazon was incredible, and the customer feedback on our products was excellent. If a hemp company is able to thread the needle by complying with Amazon’s requirements and also serving consumers with high-quality products and transparent information, then I recommend selling qualifying products on Amazon.”
However, Kelly did address some of the downsides they experienced:
“Our primary complaint about selling with Amazon is the inconsistency with which they flag and enforce guidelines. Elements they identified as unacceptable for our listings were displayed on many other brands’ products. We felt they employed selective enforcement that benefitted high-volume, cheap overseas products, and negatively targeted our small domestic company.”
In fact, Forge Hemp has now been removed from Amazon. Their seller account was frozen after they attempted to list CBN tablets (since CBN is openly sold by others), asking the Restricted Products Team for confirmation before shipping anything to Fulfilment by Amazon. Instead, their whole account – including their approved CBD topicals – was removed from their platform and their appeal has since been rejected.
The anonymous seller said the experience had been, “Rocky. It seems like the more questionable the product the longer it stays up there. We have been kicked off several times and have to repeatedly appeal being on their platform.”
Overall, those with experience selling on Amazon are not exactly positive about it. The only positive comment we received was from a company that ran into issues trying to sell their legitimate hemp products while illegitimate products remained on the storefront throughout.
What’s It Like to Buy Hemp on Amazon?
Buying hemp on Amazon might seem like one of the most convenient ways to get your cannabinoid fix, with the backing of one of the most well-established online marketplaces. However, this couldn’t be further from the truth, and not only because testing shows that the majority of products have no cannabinoids and a third don’t contain hemp at all.
Unresponsive Customer Service
After spending some time searching for contact information for the companies included in this analysis, we contacted each and every phone number, email address and contact form we were able to find.
In total, 89% of companies did not provide a responsive phone number. Of these, 9 companies provided a phone number, but did not answer our calls and so it is not clear that the number even leads to them. While 50% of companies had contact forms or email addresses, none of these responded to our inquiries.
In short, there is no way to reliably get in contact with the companies behind 89% of the products included in this analysis.
Unreliable Reviews
Reviews for hemp products on Amazon.com were often not considered to be reliable by FakeSpot, a service from Mozilla which uses AI to help determine the reliability of customer review scores.
Specifically, products had an average of 58% reliable reviews, 46% had an anomalous review history or altered reviews, and 48% were graded a D or lower for overall reliability. Note that only 48 products had enough reviews for analysis, and the number checked for anomalous history (e.g. sharp increases or declines in the number of reviews at specific times) was lower because a longer time-span is needed to make this determination.
Overall, then, this makes the usual step of checking customer reviews before buying a little challenging. You have about a 50/50 chance of being misled in one way or another by the reviews on the hemp products we looked at, and if you don’t use a tool like FakeSpot or personally analyze the reviews, it’s very difficult to know whether you’re looking at reliable or unreliable reviews.
Where Does the Hemp Come From?
Finally, despite promises from many products that they contained US-grown hemp, none of the manufacturers we reached out to who were still selling on Amazon were able to confirm this.
In fact, the only confirmation we had was from Forge Hemp, whose account and seller status has been revoked by Amazon. However, given the complete lack of communication from most other Amazon hemp brands and the fact that over a third of products don’t contain hemp at all, it’s a little difficult to judge how reliable these claims are.
How Did It Get This Bad?
With our report showing impossible dosage claims, unapproved medical claims, no hemp at all in a third of products tested, misleading reviews and a complete lack of customer support, all against the backdrop of CBD being supposedly banned from Amazon, one unavoidable question emerges: How could it have gotten this bad?
Why Can You Find CBD On Amazon at All? Should We Blame Amazon or LegitScript?
Amazon’s policy states clearly that CBD isn’t allowed, but yet we were able to find 17 products that did (as confirmed by lab testing), and all 56 at least insinuated that they may contain CBD. Moreover, this isn’t the first time this policy has been shown to be poorly enforced.
Amazon uses three examples in its CBD policy: “full spectrum” hemp, “broad spectrum” hemp and “Products that have been identified as containing CBD by LegitScript.” The first two are obvious because both are equivalent to literally just coming out and saying “contains CBD.” So given that companies are smart enough to not actively announce that their products contain a banned component, this naturally raises questions about LegitScript and the effectiveness of their service.
CBD Oracle approached David Khalaf, senior communications manager at LegitScript, to try to get to the bottom of this. He described their certification process for CBD:
“Products eligible for certification must comply with state-level regulations as well as FDA, USDA, FTC, and DEA regulations and guidances. At this time, products eligible for certification include: topical oils, cosmetics, bath products, creams, balms, salves, and whole hemp flower.
Product types that are currently not eligible for certification include: ingestible products, vapes, supplements, drugs, and pet products. Oil and tincture products may not be eligible for certification if they are intended to be taken orally or sublingually.
Furthermore, any product intended for medicinal use or used to affect the function or structure of the body must be reviewed and approved by the FDA.”
However, when we introduced our questions with, “We noticed that Amazon uses your service to determine which products contain CBD and are therefore only allowed on the storefront in very limited circumstances…”
David responded that, “I’m not aware that Amazon requires LegitScript Certification to vet CBD sellers,” clarifying on follow-up that, “I didn’t say we didn’t work with Amazon. I’m just not aware that they recognize our certification program to vet CBD products. We do perform a monitoring service for Amazon, but I’m unable to elaborate on the scope. I’d encourage you to reach out to them for details.”
Unfortunately, while we did reach out to Amazon, we did not receive a response to our inquiries. We asked David about a specific product, which clearly does not meet the guidelines he laid out for certification, and he commented that:
“While I’m unable to comment on specific products, you’re right in that any ingestible product would not qualify for certification at this point as these products generally have not been approved by the FDA. And, likewise, any product making claims that it can treat, cure, prevent, or mitigate disease would not qualify unless the sellers were willing to remediate their marketing to remove these claims.”
And yet this product remains on Amazon’s storefront today. This leaves us with the same mystery: is LegitScript missing these products or does Amazon simply not act on the information or recommendations they provide? We don’t know the extent of their collaboration, but we do know what LegitScript looks for and that Amazon claims that LegitScript identifies products that contain CBD.
To be clear, the facts are:
- Amazon works with LegitScript to determine which products break store policy by containing CBD
- Lab testing confirms that 17 of 56 tested products contain CBD and all but one of these remains on the shelves.
And this must imply one of the following conclusions:
- LegitScript is incapable of (or at least very bad at) determining which products contain CBD
- Amazon does not act on information given to them by LegitScript about products that are likely to have CBD
- Amazon does not receive much information from LegitScript and so is not aware of the multitude of offending products on its storefront
- Or some combination of these
It’s not possible to say which of these are accurate, but if we make one straightforward assumption, it does push clearly in one direction.
First off, only 2/56 products included in this investigation (the two topical creams) could possibly be certified by LegitScript, since the rest don’t meet the criteria David laid out. So the remaining issue is whether LegitScript is competent enough to determine that a product claiming to contain “3,500,000 mg” of “hemp extract” and listing “soothing and calming” and “[helping] you fall asleep and stay asleep” as benefits is likely to contain CBD.
We have to assume that no self-declared specialists in identifying disguised CBD listings could possibly miss the red flags here.
So that would lead to one conclusion: LegitScript is likely providing Amazon with information about which products contain CBD and which don’t, on an ongoing basis. However, many products still remain on the shelves today, under the same listings that in some cases have been active for over three years.
Based on all of this, we must conclude that Amazon has either been made aware of products and has failed to act on the information, or that their listed policy mischaracterizes the nature of their collaboration with LegitScript.
To be clear, our guess is that Amazon simply has not allocated enough manpower or resources to act on the information provided to them by LegitScript as part of the monitoring service they have confirmed that they provide. This is because of our conversation with LegitScript, the lack of response from Amazon and the problem we’re moving onto next.
Reporting Products to Amazon a.k.a. Wasting Your Time
Given that we identified 17 products containing CBD and many, many others that made obviously false claims and even made unapproved medical claims, you might assume that informing Amazon would fix the issue. And you would assume wrong.
On January 8th, 2024, we reported this product directly to Amazon. It claims to contain 4.5 kg (yes, really) of hemp extract and the whole product is listed as weighing 0.363 kg. A customer service representative from Amazon responded: “I will forward this right away to the higher concerned team and make sure that necessary action will be taken on this. And make sure that this will not happen with any other product.” We left an email address and requested an update on the action taken.
Two months later, the product is still available for purchase, and we received no follow-up. We also reported this same product and one other using the “Report an issue with this product or seller” link a week later, and they also remain on the storefront.
This matches the experience of Jan Brandrup, CEO of Neurogan, who told us that, “When we tried to contact Amazon about an interesting product, which is a jar weighing 33 pounds, we only got a typical response: ‘they are not the seller and recommend contacting the seller directly.’”
What does all of this tell us about Amazon? It means that in the face of obviously credible reports of false advertising on its platform, there is no action taken. Customers are being deceived, and even with no work on the part of Amazon, the issue has been identified and reported to them. And they have taken zero action on this.
As of the publication of this article, we will report additional offending products to Amazon (as well as making other, more serious reports) and create a list of these reported products. If you want to see how Amazon deals with these reports, you can check this list to see how many are still available by the date you’re reading.
This raises the question: if we weren’t a CBD and cannabis website, and we were actually LegitScript making reports, would the response be different? It’s impossible to know but given the state of the hemp market on Amazon, we can certainly make a guess.
The Lack of Enforcement Undermines the Hemp Market on Amazon
This all paints a picture which is worth spelling out explicitly: it appears there are very few substantial enforcement actions taken against sellers of obviously misleadingly marketed hemp products or those that have been shown to break Amazon’s policy.
There may be many reasons for this, but it’s hard to ignore the possibility that this is because Amazon has a financial incentive to turn a blind eye to these products and keep taking their 15% cut. We put this point to Andrew Livingston from Vicente, but he made a good point in response:
“I do not believe the prevalence of potentially intoxicating hemp cannabinoid products on Amazon is due to a conflict of incentive but more likely due to incomplete enforcement and the unclear language that these companies use for their ‘hemp oil’ gummy products. Amazon is a multibillion-dollar company that sells millions of products. The total retail value of the deceptively branded hemp oil supplement market is just a drop in a swimming pool. I think the issue is likely caused by companies using terms ‘high potency hemp oil’ rather than clearly saying CBD or other cannabinoids prohibited by Amazon’s policies.”
Andrew went on to point out that although there is an insinuation of cannabinoids, it could be that these contain hemp seed oil (which was legal before even the 2014 Farm Bill) which is allowed on Amazon. From Amazon’s perspective, then, it could simply be that hemp is a small part of their business and the issue just isn’t clear-cut enough to take action on. Everything seems to be fine on the face of it so maybe it’s just not worth investigating.
There are some issues with this explanation, although the core – Amazon just doesn’t care that much about these products – is likely true. Firstly, why hire LegitScript to identify CBD products (or at least claim to have) if you didn’t intend to prevent products from circumventing your rules? Secondly, while there is plausible deniability for the presence of CBD, there is no such deniability for products where the contents are physically impossible (3.5 kg of hemp in a 0.3 kg package) or those making medical claims.
And thirdly, if they were just essentially leaving the sellers alone in any cases of uncertainty, why do honest Amazon sellers complain about enforcement on their products while dishonest products remain on the storefront?
As an anonymous seller commented to us, “It seems like the more questionable the product the longer it stays up there. We have been kicked off several times and have to repeatedly appeal being on their platform.”
With Kelly Lombard saying, “In our experience, Amazon flags and restricts products and brands via automation, then it’s on the seller to contact a human to sort out why and get back into compliance. Our primary complaint about selling with Amazon is the inconsistency with which they flag and enforce guidelines.”
Amazon’s Enforcement Problems Extend Beyond Hemp
Of course, hemp products aren’t the only questionable or outright illegal products that remain on Amazon. The FDA explained to us that:
“The FDA has issued several warning letters to Amazon for introducing unapproved drug products and/or prohibited foods into interstate commerce. For example, the FDA issued warning letters against Amazon for the illegal sale of unapproved ophthalmic drug products as well as products labeled as energy enhancing supplements or food. Retailers, including online marketplaces, that sell and/or distribute FDA-regulated products are responsible for ensuring the products are in compliance with federal law. The FDA continues to urge stores, websites and online marketplaces, like Amazon and eBay, to protect the American public by not selling or facilitating the sale of products that violate the FD&C Act.”
While the examples the FDA mentioned to us here appear to have been cleaned up by Amazon, even warnings from them have been ineffective in the past. For example, in December 2020 they warned Amazon about male enhancement and weight loss products that contain hidden ingredients. They updated the post seven months later, noting that these products were still available for sale.
The INFORM Consumers Act (And How Amazon Is in Violation)
The INFORM Consumers Act was largely created to protect consumers against third-party sellers operating on marketplaces like Amazon, eBay, and Walmart selling stolen, counterfeit or unsafe products. The law basically addresses the anonymity sellers on stores like Amazon had previously, making the platform collect, verify and disclose information about them. In this way, third-party sellers can be held accountable for any issues with products and report any offending products.
The Key Provisions
The most important provisions of the INFORM Consumers Act are that any “high volume third party seller” on an online marketplace must provide the marketplace with:
- Bank account information
- Contact information
- Tax ID
- A working email address and phone number
And this must be confirmed at least once per year. If the information is not provided, their account must be suspended after 10 days. The marketplace must also verify this information within 10 days of receiving it.
A high volume third-party seller is defined as any one which has sold 200 or more new or unused consumer products and taken at least $5,000 in revenue in any continuous 12-month period in the last 24 months.
For sellers that make more than $20,000 in revenue in the same period, the bill also requires that some information is disclosed to consumers in a “clear and conspicuous manner,” including:
- The full name of the seller or the company name
- The physical address of the seller
- Contact information for the seller (i.e. a current working phone number or email address) “to allow for the direct, unhindered communication with high-volume third party sellers by users of the online marketplace.”
There are limited exceptions to this in some cases, for example you don’t have to share an address if you don’t have a business address and you don’t have to provide a phone number if your only phone number is a personal number.
However, in all cases, a seller with over $20k in revenue has to provide a current, working email address or phone number that allows for “direct, unhindered communication” with them, and this information must be either on the product page or on order confirmations.
Each individual violation of this law carries a penalty of $50,120.
Amazon’s Probable Violations – Up to $1.3 Million in Penalties
For the products included in our research, none provided contact information on the store page, and the order confirmations didn’t include them either.
One example is this product from Healthergize. First off, the product listing describes neither a phone number nor an email address, and the order confirmation didn’t contain one either. It does contain a link to the storefront page for the seller, but this page also doesn’t contain any contact information from the seller. We were able to find an email address on the company’s independent website, but they did not respond to our email.
Amazon lists a number of products “bought in the last month” and although it doesn’t include the usual “first listed” date, reviews for the product go back to January 2023. So with over 1,000 bought in the past month at a price of $26.09 per sale, they have clearly exceeded the $20k annual revenue threshold (and likely in the last month alone). This means that Amazon is legally required by the INFORM Consumers Act to provide either an email address or phone number which allows customers to have “direct, unhindered communication” with the seller.
This was not done, and the email address we found on another platform did not allow direct, unhindered communication. Under the rules of the INFORM Consumers Act, this must be provided, and so failing to provide it is a violation of the law and comes with a penalty of $50,120.
Before we go further, it’s important to note some limitations of how we’re estimating revenue. Amazon provides the “sold in the last month” figure and there are some complaints of inaccuracy (although this is usually that sales figures are too low), and we assumed this figure was consistent throughout the time the listing has been up. Overall it’s likely that we are over-estimating revenue somewhat, but in many cases just a few months at the listed sales level would put them over $20,000 per year.
That said, based on our estimate, 28 products crossed the $20k revenue threshold, and none of these provided contact information on the product page or the order confirmation. Two of these did have responsive customer service phone numbers, but neither of these were found on Amazon’s website, and one of these products has since been removed.
This means that the maximum potential fine for Amazon from these violations is $1,353,240.
What We’ve Done
Identifying the issues is just the first step. We asked Rod Kight, a cannabis and hemp attorney who blogs at Kight on Cannabis, about what can be done to combat the absurd dosage claims and obvious false advertising:
“These claims are likely violations of FTC rules, and reporting them to the FTC (not to mention Amazon) is a good start. Filing a lawsuit is also possible, though it may end up being a money pit as some of these companies are undercapitalized and can easily disappear or not have the funds to satisfy a judgment.”
For the unapproved medical claims, he added that, “these companies can be reported to the FDA and the FTC for their claims.”
Reporting INFORM Consumers Act Violations to the FTC
The violations of the INFORM Consumers Act that we have identified have been reported to the FTC. The Act exists to give consumers the power to hold deceptive sellers to account, and to provide a mechanism for people to have direct communication with the organization which sold the product. By not providing this information on sellers with over $20k in annual revenue, Amazon is allowing these sellers to offer often-misleading and inaccurately-labeled products to consumers with little to no accountability.
We will update this with any outcome of the reports.
Reporting Unapproved Medical Claims to the FDA
Likewise, while we do personally believe that CBD has medical benefits, insinuating to consumers that a product can cure medical conditions without adequate evidence is illegal for a reason. Customers can find their own information and make up their own mind about the potential medical benefits of CBD, but people profiting from the sale of CBD should not do so on the basis of unsupported medical claims. Just like it’s wrong for me to sell you an expensive jar of placebos and tell you it cures cancer, it’s wrong to sell CBD on the premise that it does something which has not been scientifically proven.
While we do have criticisms of the FDA’s overall lack of enforcement and regulation when it comes to CBD and hemp, we do support any efforts they make to prevent the sale of any product on false grounds.
Again, we will update this post with any actions taken as a result of our reports.
It’s Not Just Amazon: eBay, Walmart and More
This report has focused on the supposed hemp products sold on Amazon, but it’s important to remember that Amazon isn’t the only company affected by this. eBay, Walmart, and Alibaba have hemp products much like those on Amazon, and in fact the exact same products in some cases. The policy on eBay isn’t as strict (either under drugs or the rules for herbal medicines) and CBD isn’t even explicitly banned, but the same poor-quality and often outright misleading products are still sold.

So while we focus on Amazon here, the same issues are present elsewhere and the conclusions are ultimately the same in those cases. Amazon isn’t the problem so much as it is a prime example of it.
Is Amazon’s Poor-Quality “Hemp” Undermining the Whole Industry?
The widespread availability of poor-quality hemp on Amazon could potentially have knock-on effects for the rest of the industry. If your first experience with “hemp gummies” is something which claims to have an absurdly high dosage but it does nothing for you (because it doesn’t even contain hemp), this could sour you on the whole concept.
We asked both hemp sellers with experience selling on Amazon and an economic expert about this issue.
The Direct Financial Impact: Over $60 Million Per Year
First off, the fact that some people buy low-quality “hemp” from Amazon directly takes sales that would otherwise go to the wider industry. Estimating this accurately isn’t really possible, as Andrew Livingston, Director of Economics and Research at Vicente, explained to us:
“Without information on sales from Amazon, and with the reality that the hemp CBD supplement market is hard to accurately quantify given the multitude of different sales channels, it is difficult to know what percentage of the hemp supplement market is sold directly from company websites, sold through alternative stores, or sold through large online retailers like Amazon.”
This means that any estimate should be taken with a pinch of salt, to say the least. However, we can make some estimates based on the “bought in the past month” figures provided by Amazon for some products included in this analysis and the total number of products available from these sellers.
Based on the information given by Amazon, and not including anything from products without “bought in the past month” figures:
- The 56 products used for the lab analysis generated sales of over $440k in the past month, based on available figures. On average, this works out to $7.8k per seller. 14 products did not have sales data and were taken to generate $0, although this is certainly an underestimate.
- This equates to around $5.3 million per year.
- The sellers of the 56 products had a total of 227 hemp products available for sale at the time of analysis.
There are some major issues with this as an analysis of the Amazon hemp market on the whole.
- Our study did not cover all of the hemp sellers on Amazon. It is more likely to be half or fewer of all sellers.
- Revenue estimates are very limited. Not only were there 14 products that did not have sales figures, the figures given are vague (e.g. “100+ sold”) and there are complaints from sellers that they are too low.
- The products with sales data are likely the ones with more sales (based on the number of reviews). So any estimate based on this probably assumes more sales per product than the true average.
However, noting these limitations, we can adjust our estimate. Since there is a lot of uncertainty, we can calculate three different cases:
- Low Estimate: Assuming we missed 25% of sellers and 20% of revenue, Amazon’s hemp would generate $36 million in revenue per year.
- Mid Estimate: Assuming we missed 50% of sellers and one third of revenues, Amazon’s hemp would generate $64 million in revenue per year.
- High Estimate: Assuming we missed 66% of sellers and half of all revenue, Amazon’s hemp would generate $125 million per year.
As Andrew also noted, the size of the overall CBD or hemp market is hard to estimate too. Using Statista’s estimate of $4.373 billion for the CBD market in 2023, Amazon could account for up to 2.9% of the total market, with 1.5% as a best estimate.
It’s worth noting that some estimates are much higher, such as this from Whitney Economics. They estimate the total demand for hemp products at $28.4 billion, so in this case Amazon would be barely a drop in the bucket, at most 0.44% of the total demand.
Reputational Damage and Other Issues
The exact size of the financial impact isn’t really clear, but the low-quality hemp on Amazon could be doing damage to the industry’s reputation that’s even harder to estimate.
In general, deceptively labeled and marketed hemp products present a significant financial and policy risk for legal businesses.
Andrew Livingston, Director of Economics & Research, Vicente
Kelly Lombard from Forge Hemp agreed that this is damaging to the industry as a whole:
“Amazon has demonstrated that they don’t understand the difference between hemp seed oil and hemp extract that contains cannabinoids. As long as sellers are vague about a product’s contents, Amazon doesn’t seem to care. This is problematic because U.S. consumers need more information about hemp and CBD, not less. Amazon’s convenience and return policy may entice more consumers to try hemp products, but if their experience is negative, that hurts the industry.”
And the seller who wishes to remain anonymous added, “Yes. 100%. People use a product and it does not work or makes them sick and then it erodes confidence in the overall market.”
Jan Brandrup, CEO of Neurogan, commented that:
“The only authentic hemp product on Amazon is hemp seed oil […]. The rest, which make up 99% of the hemp products listed, are not related to real hemp. They are often chemical mixtures, candies, or low-quality creams with pesticides, misleadingly labeled as ‘hemp products.’”
Andrew Livingston gave a detailed run-down of the issues these types of products create:
“Low-quality, mislabeled, misleading, and products using shapes and images attractive to children negatively impact the reputation of the broader hemp products industry. Products that suggest they are intoxicating or high potency when they only contain hemp seed oil turn consumers away from engaging with the category more broadly and make it harder for legitimate companies to sell their products. Unscrupulous operators that sell products with shapes and colors that appear attractive to children only make it harder for regulated businesses to operate in legal hemp markets.”
“In general, deceptively labeled and marketed hemp products present a significant financial and policy risk for legal businesses. These products, whether they are sold on Amazon, online, or at a local convenience store are likely not produced up to the health standards consumers expect of ingestible products. They may vary significantly in their cannabinoid potency and some even contain intoxicating levels of THC.”
“It is hard to know how large their impact is financially, but I see two major risks they present for the industry. One, these products drive consumer[s] away from the entire hemp supplement category for fear that the entire market cannot be trusted or is not properly regulated. This means legitimate businesses with great products that benefit consumers lose out. Second, problematically labeled and branded hemp products indicate to regulators and public officials that the entire category needs more robust and stringent restrictions. But the products that incite this desire for more regulation often exist outside of the legal system thereby creating additional requirements for already compliant businesses without the necessary enforcement against those operating outside of the rules.”
In short, everyone we spoke to agreed that these products are bad for the industry as a whole, not just for the reputation of the hemp companies on Amazon. They drag everyone down along with them.
How to Improve the Situation for Hemp on Amazon (and Other Marketplaces)
To put it bluntly, the hemp situation on Amazon is grim. Unreliable brands make truly absurd claims and cover their flagrant violations of store policy with the paper-thin disguise of being “hemp” and not anything specifically banned. It’s likely that Amazon – for whatever reason – is unable to enforce sufficiently to make any meaningful dent in the widespread rule-breaking.
And if you’re just buying hemp gummies, it probably won’t contain cannabinoids and in about a third of cases it will just be a pack of ordinary gummies with a high price tag and no hemp at all. If you’re “lucky” it will likely just be substantially less CBD than you assumed you were getting.
So how can we improve the situation? Mike Sill from Sunday Scaries wrote a great LinkedIn post about this issue, but gave a run-down of his main suggestions to CBD Oracle in an email:
“Amazon should then ban the illegitimate brands that falsely advertise how much CBD is in their products or that make false medical claims.
In order to separate which brands are legitimate or not, Amazon should have a verification process that includes the following:
- The seller uses manufacturing facilities that are FDA-registered and GMP (Good Manufacturing Processes)-certified.
- The seller should have and publish legitimate third-party Certificates of Analysis (COA) that ensure products are precisely what they say they are—at the correct dosages.
- The seller should produce reviews and testimonials from verified buyers on their actual web domains. These reviews should come from trusted review tools like Okendo, Trustpilot or Yotpo.
Fly-by-night, brand-burning companies don’t have many verified reviews because every time they spin up a new label, they start from scratch. Or, the Amazon reviews are fraudulently purchased. [For example] the illegitimate brand will pay an overseas company to purchase the product and write a review, and then reimburse the company for the costs.”
Kelly Lombard added that more engagement with the hemp industry could solve the problems Amazon is facing:
“I would love it if Amazon would bring in representatives from the hemp industry to evaluate the products currently available on their site and make recommendations to strengthen enforcement or their stated policies, or perhaps identify a pathway to expand hemp offerings. I don’t think Amazon will choose to do this because they’re making plenty of money on the current system and aren’t burdened by additional accountabilities.”
Jan Brandrup from Neurogan had some helpful suggestions for customers too:
“I suggest that Amazon should implement due diligence. Customers should be encouraged to research the company’s background and request recent lab reports to check product consistency. I strongly advise all Amazon shoppers, whether buying supplements or hemp products, to ask for updated lab reports. Comprehensive testing is surprisingly affordable today, around $80-$90 for a complete test (full-panel test). Products should be required to have a batch number and a lab report showing potency and purity. Buyers must stay alert, as all products are made in China. You could buy a million gummies for about $0.01 each, repackage them in a warehouse, add fancy labels, and falsely market them as hemp products.”
If you’re reading these suggestions with a lingering sense of déjà vu, that’s because they fall right in line with what people have been arguing for as long as the hemp industry has existed. Bad actors always try to weasel their way into industries with unclear rules and lots of profit to be made, and CBD and hemp more generally have been this way since the Farm Bill was passed without substantial regulations in place.
Websites like CBD Oracle and many others have long called for all brands to make lab tests available to consumers and for all consumers to only shop from brands that provide these COAs. Products should be made in GMP-certified facilities and the dosage on the label should correspond to that found in the product itself. The problem for the industry, and the problem for Amazon now, is that these have not been required in any substantial way.
For the industry on the whole, the widespread competition and increasing state-level regulation has pushed manufacturers in the right direction. For Amazon, their supposed “no CBD” policy makes a similar push in the right direction very difficult to achieve. If you’re selling CBD somewhere that CBD isn’t allowed, it would obviously be financially suicidal to release evidence of this in the form of a lab report, for instance.
Mike Sill explained the necessary first step very clearly:
“Amazon should recognize that they are currently allowing CBD to be sold on their platform. This is against their policy, but brands are circumnavigating the rules by masking the CBD products as “hemp” on the labels. Addressing that they have this problem is the first step.”
If Amazon doesn’t acknowledge the reality of the market they created, they cannot take all of the sensible steps proposed by Mike and others. Of course, one “simpler” solution for Amazon would be to simply locate and remove products which break their rules, and indeed those with more serious violations such as unapproved medical claims. They would have to continually monitor the “hemp” market on the platform and request lab reports for any products insinuating the presence of cannabinoids.
While this solution would keep the rules consistent and improve the situation in a sense, a much better solution is to embrace the market but ensure that standards are high and laws are followed. Whether they like it or not, there is CBD on their platform and it’s unlikely they will hire enough people to check every new listing to prevent offending products from being sold.
With that in mind, a better solution would be:
- Immediately remove all products making obviously false claims about the quantities of hemp they contain or that make medical claims. This is a no-brainer: customers should not be willfully deceived.
- Loosen the prohibition on selling CBD. Amazon doesn’t have to jump feet-first into the industry; they could keep restrictions on any products containing detectable levels of THC, for instance, or disallow edible products because they are outlawed by the FD&C Act.
- Ensure that all CBD and hemp companies on the platform have a legitimate business that is based in the US. This would ensure that customers (and Amazon themselves) can hold them responsible if anything goes wrong, and if any laws are broken. This would also strongly encourage companies to use US-grown hemp in their products.
- Require that all sellers use GMP-certified facilities and provide third-party lab reports to consumers, from ISO/IEC 17025:2017 accredited labs. These two pieces of documentation alone would go a long way to ensuring that the quality of the products on Amazon is high.
- Make companies update lab reports on an ongoing basis, at least once per quarter, and remove or re-label any products where the lab report shows more than 10% variation from the stated dosage on the container.
It might seem strange that the solution to low-quality CBD-containing products would be to loosen restrictions on CBD, but the current rules essentially incentivize lying, and thereby produce a market where the liars rule the roost. If you can’t completely stop people from lying, you can at least give space to those telling the truth.
Opening up to legitimate CBD sellers but also raising the bar when it comes to transparency and quality would correct most of these problems. If a seller had to produce a COA before listing a CBD product, 94.6% of products we investigated would have not been able to get onto the storefront at all. If these COAs had to show that customers were getting what it said on the label, every single product that promised over 1 million mg of “hemp extract” would have never made it to the storefront.
Conclusion – Amazon Is Failing Its Customers and Likely Breaking the Law
It is hard to be too harsh on Amazon for the current situation with hemp on their platform. An ill-thought-out blanket ban on CBD created a vacuum in which only the most dishonest of companies could succeed, and efforts to stamp out deceptive or violating products have been lackluster at best. Even directly reporting products to Amazon whose claims contradict the laws of physics is totally useless.
Just like the traveling snake oil salesmen peddling ineffective products based on lies and leaving town before anybody could ask for their money back, the hemp sellers of Amazon make bold claims with no regard for accuracy and instead depend on insinuation and shills in the review section to make their products appear legitimate. And just like those snake oil salesmen whose products often didn’t actually contain snake oil, the hemp oil salesmen of Amazon make tens of thousands of dollars selling products that often don’t actually contain any hemp at all.
We learned the lessons from those snake oil days. We make sure that anything purporting to be medicine is backed up with scientific evidence and we intrinsically distrust anybody who rolled into town two days ago promising a cure for all of our ills. Our medicine is sold by pharmacies and kept in check with rules, regulations and requirements that protect both the customers and the sellers themselves. The snake oil sellers could never compete with real medicine, even if they tried.
And that is what Amazon needs to do to its hemp market. Even establishing a bare minimum requirement for hemp sellers – showing an up-to-date lab report – would be enough to send the snake oil sellers running for the hills. Will you be able to pretend that CBD isn’t available on your platform? No. But customers who are buying CBD on your platform – who already exist, like it or not – would be much, much more likely to get safe products that offer what they say on the label.
Amazon did not respond to multiple requests for comment on the findings in this report.
Data Availability
The full dataset from the analysis is available in this spreadsheet. You can download a summary of the report in PDF here.
If you have any questions or feedback about the data, contact us at [email protected].
Methodology
The lab testing was conducted by Infinite Chemical Analysis Labs, a California-based laboratory accredited by the Department of Cannabis Control (DCC) and ISO/IEC 17025. Full details of the methodology are available here.
In brief, the products were tested for cannabinoid potency, and those coming back negative were also tested to determine whether they contained hemp oil of any type. A subset of products were also tested for pesticides, mycotoxins, heavy metals, residual solvents, microbials and foreign materials.
The specific analytical method varies depending on what the lab is looking for. The main approaches used are:
- Potency: Ultra high-performance liquid chromatography coupled with a diode array detector (UHPLC-DAD). The samples were prepared by cryo-grinding, blending or milling to a homogeneous consistency, then mixed with an appropriate solvent. The concentrations are determined against a calibration curve.
- Pesticides and mycotoxins: Detected with a combination of gas and liquid chromatography triple-quad mass spectrometry (GC-MS/MS and LC-MS/MS). Again, the material is homogenized before being injected into the instrument and compared to a calibration curve.
- Heavy metals: InfiniteCAL looked for arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) using inductively-coupled plasma mass spectrometry (ICP-MS). After homogenization, weighing and digestion with nitric acid, analyses are conducted in kinetic energy discrimination (KED) mode with helium as the collision gas and argon as the carrier gas.
- Residual solvents: Residual solvents were detected using headspace gas chromatography single-quad mass spectrometry (HS-GC-MS). Again, this is calibrated using certified reference material standards.
- Microbials: InfiniteCAL used real-time polymerase chain reaction (qPCR) to determine whether pathogenic microbes were present. Aliquots (i.e. smaller sub-samples) are drawn from the homogenized sample, enriched with a broth and then left for 24 hours to encourage microbial growth. The cells in the resulting sample are lysed and the DNA extracted, before being probed for the presence of target microbes.
- Foreign materials: Samples were analyzed visually and with a digital microscope magnifier for the presence of insect fragments, hairs, mammalian excreta, mold, sand, soil, cinders and dirt.
- Hemp oil: InfiniteCAL used gas chromatography coupled with mass spectrometry (GC-MS) to determine whether the samples contained hemp oil (this was only performed for products showing no cannabinoids). Samples were homogenized as described above (under “Potency”), filtered and added to vials before being injected into the instrument. The resulting chromatograms are analyzed for hemp oil compounds like triglycerides and diglycerides by comparison with a hemp oil standard.
References
- Amazon. (2024). Drugs and Drug Paraphernalia. Amazon.com. https://sellercentral.amazon.com/help/hub/reference/external/G200164490
- Conway, J. (2023). CBD product Dollar Sales U.S. 2022-2026. Statista. https://www.statista.com/statistics/1067467/cbd-product-dollar-sales-us/
- Center for Food Safety and Applied Nutrition. (2002). Small entity compliance guide on Structure/function claims. U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-structurefunction-claims
- Food, Drug and Cosmetic Act, 21 U.S.C. ch. 9 §331 https://uscode.house.gov
- Greene, J. (2019). Amazon prohibits CBD sales, but it’s still easy to buy on the site. Washington Post. https://www.washingtonpost.com/technology/2019/12/19/amazon-prohibits-cbd-sales-its-still-easy-buy-site/
- INFORM Consumers Act, 15 U.S.C. § 45f (2022). https://uscode.house.gov
- Johnson, L. (2023). Is delta-8 THC legal? A state-by-state analysis. CBD Oracle. https://cbdoracle.com/news/policy/delta-8-thc-legal/
- Sill, M. (2022). Don’t buy CBD on Amazon (yet)! here’s why. LinkedIn. https://www.linkedin.com/pulse/dont-buy-cbd-amazon-yet-heres-why-mike-sill/
- Staff in the Office of Technology and The Division of Privacy and Identity Protection. (2023). Informing businesses about the INFORM Consumers Act. Federal Trade Commission. https://www.ftc.gov/business-guidance/resources/INFORMAct
- Venable, LLC. (2014). Permissible vs. impermissible structure/function claims for Dietary Supplements. Venable. https://www.venable.com/files/upload/FDLI-Dietary_Supplements.pdf
- Whitney, B. R., & Wilberding, B. (2023). 2023 U.S. National Cannabinoid Report. Whitney Economics. Retrieved from https://www.whitneyeconomics.com/2023-us-national-cannabinoid-report.
- Lombard, K. (January 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Kelly-Lombard-Forge-Hemp-comments-Amazon-hemp-market.pdf
- Kight, R. (December 2023). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Rod-Kight-comments-Amazon-hemp-market.pdf
- Paulson, E. (February 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Erik-Paulson-PhD-comments-Amazon-hemp-market-study.pdf
- Sill, M. (January 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Mike-Sill-Sunday-Scaries-comments-Amazon-hemp-market.pdf
- Livingston, A. (January 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Andrew-Livingston-comments-Amazon-hemp-market.pdf
- Brandrup, J. (January 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Jan-Brandrup-Neurogan-comments-Amazon-hemp-market.pdf
- Anonymous seller (January 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/Anonymous-seller-comments-about-Amazon-hemp-market.pdf
- Khalaf, D. (January 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/David-Khalaf-LegitScript-comments-Amazon-hemp-market.pdf
- The Food and Drug Administration (February 2024). Email interview. https://cbdoracle.com/wp-content/uploads/2024/03/FDA-comments-Amazon-hemp-market.pdf
Acknowledgments
We would like to thank Kelly Lombard, Erik Paulson, Mike Sill, Andrew Livingston, Rod Kight, Jan Brandrup, David Khalaf, and the Food and Drug Administration (FDA) for providing comments and guidance for the report. Without your help, this would not have been possible.
Editor’s note – March 26, 2024: Many sellers from this study tweaked their Amazon product page URL after this report went live and as a result, some of the original product links in the datasheet led to 404 pages. We added a new column to this sheet to include some of the updated product page links for these products.

