Cannabicyclol (CBL) is a super-rare, non-intoxicating cannabinoid that results from the breakdown of cannabichromene (CBC). Purified CBL is synthetic and only available to researchers, not consumers.
Data are sparse, but CBL could be a weak anti-inflammatory, anti-cancer, and antimicrobial agent. The side effects are entirely unknown but probably reflect the most commonly known ones of hemp.
What Is CBL?
Cannabicyclol, or CBL, is literally an ancient cannabinoid that remains shrouded in mystery despite being related to major cannabinoids. Recent archeological evidence found CBL present in 2700-year-old cannabis, but it technically wasn’t discovered and synthesized until the late 1960s.
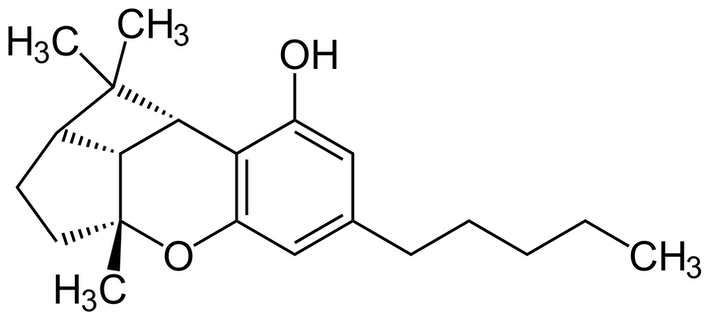
CBL is made when CBC, or cannabichromene, is degraded (oxidized) by light, heat, and air over time.
CBL is a structural isomer of many cannabinoids we know, including THC, CBD, delta-8 THC, and CBC. This means they are all made up of the same parts that are just rearranged. These days, CBL must be synthetically made for study because it can’t be significantly extracted for commerce.
Importantly, CBL doesn’t have an intoxicating double bond like THC does. This atomic change results in significant differences between them, such as in psychoactivity and receptor targets.
CBL is believed to partly interact with our endocannabinoid system (ECS), and certainly with the larger endocannabinoidome.
CBL vs. THC: Key Differences
- THC is the main psychoactive component of cannabis and causes a high, while CBL is non-intoxicating and may be more likely to be found in hemp and CBD-dominant plants
- THC’s main target is the classic ECS: CB1/CB2 receptors and enzymes
- CBL’s profile likely has some ECS activity and acts in the endocannabinoidome (eCBome) sphere of influence
- THC is abundantly harvested, extracted, and heartily available in state-legal areas, while CBL requires synthesis and remains for research use only
CBL vs. CBD: Key Differences
- CBD is the most abundant non-intoxicating cannabinoid in such plants. Meanwhile, CBL is among the least common and necessitates artificial production.
- CBD ranks within the gold standard of medical research (e.g., Epidiolex with FDA approval) and has many well-supported clinical and anecdotal uses
- CBL is treated as an oxidative contaminant that facilities try to avoid accidentally creating, especially when drying plant material
- CBL is a lesser anti-inflammatory agent than CBD
What Are the Benefits and Effects of CBL?
Learning the benefits of CBL is in the very early stages for researchers. Animal studies happened in the 70s and there are a few newer bench studies, but no human trials have been done. So far, CBL may be beneficial for:
- Anti-inflammatory: Albeit weak, CBL has the right structure for it but the concentration of CBL needed to stop inflammatory prostaglandins is reportedly less than all other cannabinoids tested in the same experiment (e.g., THC and isomers, CBD, CBC)
- Anti-cancer: CBL has anti-proliferative activity on human colon cancer cells in test tubes
- Antimicrobial: Again, CBL showed almost no MRSA inhibition, but the general antibiotic potential is there
Comparable non-psychoactive cannabinoids like CBD are known for being anticonvulsant, vasorelaxant, pain-relieving, antianxiety, antiemetic, neuroprotective, and more. The degree to which CBL shares these benefits is to be determined.
What Are the Side Effects of CBL?
In 1976, a flawed experiment serially inbreeding rabbit generations with known autosomal mutations for convulsions was performed to assess any pro-convulsive effects of cannabinoids. The rabbits were surgically implanted with IVs in the jugular, and then given high doses of cannabinoids including CBL, amphetamines, or hallucinogens.
Unsurprisingly, one of them died of a blood clot in the lung just 7 minutes after receiving 8 mg/kg of CBL by jugular IV. This death was most likely related to extensive inbreeding, surgical manipulation, IV administration, and underlying genetic anomalies.
To this day, convulsions and blood clots are not known nor documented side effects of cannabinoids. They’re usually the opposite.
- Side effects of CBL in humans and animals are still not accurately known or determined
- CBL likely shares the side effect profile of similar cannabinoids like CBC and CBD
Dosing: How Much CBL Should You Take?

CBL by itself is not available in everyday hemp or cannabis products, and its safety and tolerability are unknown. To date, there is no evidence or indication to justify taking a highly experimental cannabinoid like CBL, even if the risk seems small.
Super-rare cannabinoids like CBL are too difficult to significantly extract from the cannabis plant, so they are custom-ordered and synthesized for lab research. Scarcity, lack of research, and no consumer demand mean that CBL products won’t be on the market for some time.
To find out which cannabinoids, doses, and routes may be right for you, speak to your healthcare provider first and consider consulting with an experienced cannabis coach.
Will CBL Get You High?

No. CBL is recognized as part of the non-intoxicating cannabinoids found naturally in hemp and cannabis. Virtually all cannabinoids that start with the letter “C” don’t have intoxicating properties.
However, CBL is believed to interact with our endocannabinoid system and could be subtly “psychoactive”. This is to say, technically, it could still positively influence your mood and well-being like other cannabinoids do (e.g., CBD, THCV) but without causing a high.
Will CBL Show Up on a Drug Test?
CBL will not likely show up on a drug test unless the sample is flagged for THC first. CBL itself may be detectable on a drug screen with a cutoff of 2 ng/mL. Since CBD metabolites are similar, we could reasonably expect very low cross-reactivity, or a tiny (1-2%) chance the lab will think CBL metabolites are THC metabolites.
However, this kind of testing is atypical, unless the lab is trying to determine herbal versus prescription cannabis use. Only 2 samples from over 1,500 urine specimens tested positive for both THC and CBL, making it difficult to use as a distinguishing biomarker.
Conclusion
Currently, we can only imagine CBL has similar effects to our favorite non-psychoactive cannabinoids. But it’s likely weaker, and CBL leaves much to be determined.
Related Cannabinoids
- Cannabidiol (CBD)
- Cannabichromene (CBC)
- Tetrahydrocannabinol (THC)
- Tetrahydrocannabinolic acid (THCA)
- Delta-8 tetrahydrocannabinol (Delta-8 THC)
- Tetrahydrocannabivarin (THCV)
References (13)
- Vikingsson, S., Hart, E. D., Winecker, R. E., Cone, E. J., Kuntz, D. J., Clark, M., Jacques, M., Hayes, E. D., & Flegel, R. R. (2023). Prevalence of ∆8-tetrahydrocannabinol carboxylic acid in workplace drug testing. Journal of Analytical Toxicology, bkad068. https://doi.org/10.1093/jat/bkad068
- Filer, C. N. (2022). Cannabinoid Photochemistry: An Underexplored Opportunity. Cannabis and Cannabinoid Research, 7(6), 725–727. https://doi.org/10.1089/can.2022.0113
- Lee, H.-S., Tamia, G., Song, H.-J., Amarakoon, D., Wei, C.-I., & Lee, S.-H. (2022). Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. International Immunopharmacology, 108, 108865. https://doi.org/10.1016/j.intimp.2022.108865
- Uziel, A., Milay, L., Procaccia, S., Cohen, R., Burstein, A., Sulimani, L., Shreiber-Livne, I., Lewitus, D., & Meiri, D. (2022). Solid-State Microwave Drying for Medical Cannabis Inflorescences: A Rapid and Controlled Alternative to Traditional Drying. Cannabis and Cannabinoid Research. https://doi.org/10.1089/can.2022.0051
- Goldman, S., Bramante, J., Vrdoljak, G., Guo, W., Wang, Y., Marjanovic, O., Orlowicz, S., Di Lorenzo, R., & Noestheden, M. (2021). The analytical landscape of cannabis compliance testing. Journal of Liquid Chromatography & Related Technologies, 44(9–10), 403–420. https://doi.org/10.1080/10826076.2021.1996390
- Scheunemann, A., Elsner, K., Germerott, T., Hess, C., Zörntlein, S., & Röhrich, J. (2021). Extensive phytocannabinoid profiles of seized cannabis and cannabis-based medicines – Identification of potential distinguishing markers. Forensic Science International, 322, 110773. https://doi.org/10.1016/j.forsciint.2021.110773
- Cerino, P., Buonerba, C., Cannazza, G., D’Auria, J., Ottoni, E., Fulgione, A., Di Stasio, A., Pierri, B., & Gallo, A. (2021). A Review of Hemp as Food and Nutritional Supplement. Cannabis and Cannabinoid Research, 6(1), 19–27. https://doi.org/10.1089/can.2020.0001
- Klahn, P. (2020). Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics, 9(6), 297. https://doi.org/10.3390/antibiotics9060297
- Yeom, H.-S., Li, H., Tang, Y., & Hsung, R. P. (2013). Total Syntheses of Cannabicyclol, Clusiacyclol A and B, Iso-Eriobrucinol A and B, and Eriobrucinol. Organic Letters, 15(12), 3130–3133. https://doi.org/10.1021/ol401335u
- Russo, E. B., Jiang, H.-E., Li, X., Sutton, A., Carboni, A., del Bianco, F., Mandolino, G., Potter, D. J., Zhao, Y.-X., Bera, S., Zhang, Y.-B., Lü, E.-G., Ferguson, D. K., Hueber, F., Zhao, L.-C., Liu, C.-J., Wang, Y.-F., & Li, C.-S. (2008). Phytochemical and genetic analyses of ancient cannabis from Central Asia. Journal of Experimental Botany, 59(15), 4171–4182. https://doi.org/10.1093/jxb/ern260
- Martin, P., & Consroe, P. (1976). Cannabinoid induced behavioral convulsions in rabbits. Science (New York, N.Y.), 194(4268), 965–967. https://doi.org/10.1126/science.982057
- Burstein, S., Levin, E., & Varanelli, C. (1973). Prostaglandins and cannabis—II inhibition of biosynthesis by the naturally occurring cannabinoids. Biochemical Pharmacology, 22(22), 2905–2910. https://doi.org/10.1016/0006-2952(73)90158-5
- Crombie, L., Ponsford, R., Shani, A., Yagnitinsky, B., & Mechoulam, R. (1968). Hashish components. Photochemical production of cannabicyclol from cannabichromene. Tetrahedron Letters, 55, 5771–5772. https://doi.org/10.1016/s0040-4039(00)76346-5
Editor’s note: This article was originally published on January 20, 2022, by Ali Mans Cornwell.

