Key Takeaways
- CBDA is the raw, unheated form of CBD, offering unique benefits distinct from its more well-known counterpart.
- It shows potential in anti-inflammatory and anti-nausea applications, interacting differently with the body’s endocannabinoid system.
- Incorporating CBDA into your wellness routine can provide a fresh, natural approach to health and well-being.
CBDA is the main cannabinoid in hemp that easily converts into CBD before and after ingestion. Thus, it carries similar properties as an anti-inflammatory, pain-relieving, antiemetic, anticonvulsant, neuroprotective, and anticancer molecule.
Since CBDA is a pro-drug to CBD, it’s expected to have similar drug interactions and side effects like GI disturbance, drowsiness, and reduced appetite. All of the above are pending clinical studies.
What Is CBDA?
CBDA, or cannabidiolic acid, is the acidic cannabinoid that gives rise to CBD through light, heat, or metabolism upon ingestion. It was first isolated in 1955, and its structure mapped in 1965.
Because of how easily the acidic group falls off, oral CBDA is much better absorbed and gives 3 times greater CBD levels than oral CBD alone.
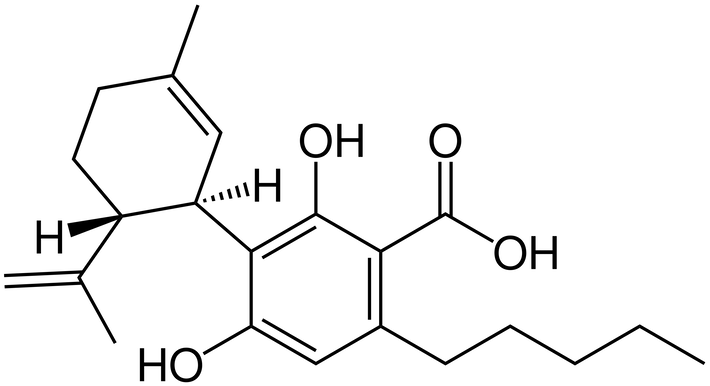
CBD products are common and shelf-stable. But CBDA products are heat-sensitive, need special handling, and rapidly break down into CBD within the product or in the body anyway. Specially formulated CBDA that is not metabolized into CBD is called HU-580 and is not clinically studied, and neither is CBDA for any condition.
CBDA vs. THC: Key Differences
- THC is the main, intoxicating cannabinoid in cannabis, and CBDA is the most abundant, non-intoxicating one in hemp
- THCA/THC dominance defines chemotype I cannabis; CBDA/CBD dominance creates chemotype III plants or what we call “hemp”
- CBDA may present a non-intoxicating alternative to THC for pain, inflammation, nausea, and seizures but there isn’t any clinical data for CBDA
CBDA vs. CBD: Key Differences
- CBDA is the most abundant and acidic cannabinoid that directly gives rise to CBD
- Oral CBDA may be around 3 times better absorbed and 1,000 times more potent at the serotonin receptor, but has no clinical data
- In mice, CBDA may reduce nausea and seizures at lower doses
What Are the Benefits and Effects of CBDA?
While pharmaceutical CBD (Epidiolex®) is FDA-approved, CBDA awaits clinical study. Based solely on animal studies and computer simulations, CBDA may have similar benefits including:
- Anti-inflammatory and pain-relieving: Via the eCBome and selective COX-2 inhibition (like the NSAID Celecoxib)
- Anti-nausea and anti-emetic: 1,000 times more potent than CBD in nausea-conditioned mice; suppresses acute and anticipatory nausea at lower doses
- Anticonvulsant: More effective at lower doses in mice
- Neuroprotective: Aside from seizures, in mice, CBDA may be a therapeutic agent in Alzheimer’s or Parkinson’s disease
- Antidepressant: More effective in male mice
- Chronic Liver Disease: Candidate for attenuating liver disorders like NALFD, ALD, and HCV-induced liver complications
- SARS-CoV-2: Computer models predict CBDA may prevent COVID-19 infection at the point of cell entry. CBD may help decrease subsequent inflammation and damage
- Anticancer: In leukemic and breast cancer cells
What Are the Side Effects of CBDA?
CBDA readily becomes CBD, so the side effect profiles are expected to be similar until formal research confirms this. Since CBDA is better absorbed, it’s conceivable that you could be more likely to run into CBD side effects than with CBD alone at lower or the same doses.
The side effects of CBDA may be approximate to CBD and include mild to moderate:
- Gastrointestinal symptoms
- Sleepiness
- Reduced appetite
- Elevated liver enzymes (LFTs)
To date, CBD shows a great safety profile. Persons taking anticonvulsant medications like clobazam and valproate may have significant medication interactions and need clinical guidance.
How Much CBDA Should You Take?
CBDA dosing should look similar to CBD dosing, but it may allow for lower dosing of either. Currently, there aren’t formally published CBDA dosing recommendations as there are for CBD.
Epidiolex® (pure pharmaceutical CBD) has specific, weight-based prescribing for children. Outside of that, expert opinions published as a Delphi consensus recommend starting CBD at 5 mg twice daily for pain in adults, with a titration plan of up to 40 mg CBD before introducing 1:1 CBD:THC.
However, some experts disagree and state those conservative recommendations aren’t based on good-quality evidence. Such dosing is also significantly lower than studied amounts of CBD for indications besides pain, so your clinician should decide case-by-case.
Therefore, whenever I suggest starting CBD or CBDA I will usually start higher at 10 mg twice daily with an equal part of CBDA (1:1 CBD:CBDA) to increase synergy, absorption, and effectiveness. This gives better “bang for your buck”, and I help people titrate from there.
Will CBDA Get You High?
No, CBDA is not a cannabinoid that can cause a high. CBDA is the non-intoxicating parent to CBD, another well-known, non-intoxicating cannabinoid. Unlike THCA which can become psychoactive THC if smoked or baked, heating CBDA won’t create a psychoactive substance.
Still, CBDA directly becomes CBD, which increases anandamide (AEA). AEA is a natural endocannabinoid responsible for elevating mood and causes the “runner’s high” after periods of intense exercise.
You won’t actually get high, but CBDA and CBD can make you feel better in other ways that don’t affect your thinking capacity or senses.
Will CBDA Show Up on a Drug Test?

No, pure CBDA should not show up on a drug test. Barring any minimal chance of errors in screening, full-spectrum product use, or unwitting THC contamination. Consumers should choose CBDA isolate or broad-spectrum, “THC-free” hemp products with non-detectable levels of THC to minimize this risk.
CBDA was not detectable nor cross-reactive in a 2023 study using six commercial drug tests nor in a 2021 forensic study. Only 7 people in a 2023 meta-analysis of over 1,500 urine samples had detectable CBD-A. Nevertheless, CBD metabolites have a very low (1-2%) chance of cross-reacting and causing a false positive.
Conclusion
Taking CBDA essentially means you’re taking a better-absorbed form of CBD, and you’ll probably get more out of it than with the same amount of CBD alone. Since CBDA is dominant in hemp food, products, and seed oil, knowing this information is important for everyday consumers.
References
- Barré, T., Di Marzo, V., Marcellin, F., Burra, P., & Carrieri, P. (2023). Expanding Research on Cannabis-Based Medicines for Liver Steatosis: A Low-Risk High-Reward Way Out of the Present Deadlock? Cannabis and Cannabinoid Research, 8(1), 5–11. https://doi.org/10.1089/can.2022.0014
- Benavides, A. (2021, October 25). All About Anandamide: Taking Cannabinoid Treatment to New Heights. Cannabis Central. https://www.veriheal.com/blog/what-is-anandamide/
- Berk, K., Bzdega, W., Konstantynowicz-Nowicka, K., Charytoniuk, T., Zywno, H., & Chabowski, A. (2021). Phytocannabinoids—A Green Approach toward Non-Alcoholic Fatty Liver Disease Treatment. Journal of Clinical Medicine, 10(3), 393. https://doi.org/10.3390/jcm10030393
- Filer, C. N. (2023). Delta-9-Tetrahydrocannabinolic Acid B: A Mechanism for its Formation in Cannabis. Cannabis and Cannabinoid Research, 8(1), 1–4. https://doi.org/10.1089/can.2021.0216
- Formato, M., Crescente, G., Scognamiglio, M., Fiorentino, A., Pecoraro, M. T., Piccolella, S., Catauro, M., & Pacifico, S. (2020). (‒)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules, 25(11), 2638. https://doi.org/10.3390/molecules25112638
- Hen-Shoval, D., Moshe, L., Indig-Naimer, T., Mechoulam, R., Shoval, G., Zalsman, G., Kogan, N. M., & Weller, A. (2023). Cannabinoid Receptor 2 Blockade Prevents Anti-Depressive-like Effect of Cannabidiol Acid Methyl Ester in Female WKY Rats. International Journal of Molecular Sciences, 24(4), Article 4. https://doi.org/10.3390/ijms24043828
- Hill, K. P., & Abrams, D. I. (2021). A cannabis oracle? Delphi method not a substitute for randomized controlled trials of cannabinoids as therapeutics. Journal of Cannabis Research, 3(1), 23. https://doi.org/10.1186/s42238-021-00074-0
- Janecki, M., Graczyk, M., Lewandowska, A. A., & Pawlak, Ł. (2022). Anti-Inflammatory and Antiviral Effects of Cannabinoids in Inhibiting and Preventing SARS-CoV-2 Infection. International Journal of Molecular Sciences, 23(8), 4170. https://doi.org/10.3390/ijms23084170
- Kim, J., Choi, P., Park, Y.-T., Kim, T., Ham, J., & Kim, J.-C. (2023). The Cannabinoids, CBDA and THCA, Rescue Memory Deficits and Reduce Amyloid-Beta and Tau Pathology in an Alzheimer’s Disease-like Mouse Model. International Journal of Molecular Sciences, 24(7), Article 7. https://doi.org/10.3390/ijms24076827
- Krämer, M., Schäper, M., Dücker, K., Philipsen, A., Losacker, M., Dreimüller, N., Engelmann, J., Madea, B., & Hess, C. (2021). Detectability of cannabinoids in the serum samples of cannabis users: Indicators of recent cannabis use? A follow-up study. Drug Testing and Analysis, 13(9), 1614–1626. https://doi.org/10.1002/dta.3110
- Madeo, G., Kapoor, A., Giorgetti, R., Busardò, F. P., & Carlier, J. (2023). Update on Cannabidiol Clinical Toxicity and Adverse Effects: A Systematic Review. Current Neuropharmacology. https://doi.org/10.2174/1570159X21666230322143401
- Mboumba Bouassa, R.-S., Sebastiani, G., Di Marzo, V., Jenabian, M.-A., & Costiniuk, C. T. (2022). Cannabinoids and Chronic Liver Diseases. International Journal of Molecular Sciences, 23(16), 9423. https://doi.org/10.3390/ijms23169423
- Souza, J. D. R., Pacheco, J. C., Rossi, G. N., de-Paulo, B. O., Zuardi, A. W., Guimarães, F. S., Hallak, J. E. C., Crippa, J. A., & Dos Santos, R. G. (2022). Adverse Effects of Oral Cannabidiol: An Updated Systematic Review of Randomized Controlled Trials (2020–2022). Pharmaceutics, 14(12), 2598. https://doi.org/10.3390/pharmaceutics14122598
- Suryavanshi, S. V., Kovalchuk, I., & Kovalchuk, O. (2021). Cannabinoids as Key Regulators of Inflammasome Signaling: A Current Perspective. Frontiers in Immunology, 11, 613613. https://doi.org/10.3389/fimmu.2020.613613
- van Breemen, R. B., Muchiri, R. N., Bates, T. A., Weinstein, J. B., Leier, H. C., Farley, S., & Tafesse, F. G. (2022). Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. Journal of Natural Products, 85(1), 176–184. https://doi.org/10.1021/acs.jnatprod.1c00946
- Vikingsson, S., Hart, E. D., Winecker, R. E., Cone, E. J., Kuntz, D. J., Clark, M., Jacques, M., Hayes, E. D., & Flegel, R. R. (2023). Prevalence of ∆8-tetrahydrocannabinol carboxylic acid in workplace drug testing. Journal of Analytical Toxicology, bkad068. https://doi.org/10.1093/jat/bkad068
- Wakshlag, J. J., Schwark, W. S., Deabold, K. A., Talsma, B. N., Cital, S., Lyubimov, A., Iqbal, A., & Zakharov, A. (2020). Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract. Frontiers in Veterinary Science, 7, 505. https://doi.org/10.3389/fvets.2020.00505
- Walsh, K. B., McKinney, A. E., & Holmes, A. E. (2021). Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Frontiers in Pharmacology, 12, 777804. https://doi.org/10.3389/fphar.2021.777804
- Wolf, C. E., Pokhai, A. A., Poklis, J. L., & Williams, G. R. (2023). The Cross Reactivity of Cannabinoid Analogs (Delta-8-THC, Delta-10-THC and CBD), their metabolites, and Chiral Carboxy HHC metabolites in Urine of Six Commercially Available Homogeneous Immunoassays. Journal of Analytical Toxicology, bkad059. https://doi.org/10.1093/jat/bkad059
